The ABPI Code in a Day
Wednesday 23 April 2025
10:00 - 16:00
Find out more
Wednesday 17 January 2024
09:00-17:00
If you are new to the Pharmaceutical Industry, this workshop is for you!
This introductory level workshop led by Dr Sheuli Porkess and Susan Tansey will support you in building wide-ranging foundational understanding of the development of new medicines and aims to aid your transition to industry.
The course has been configured for digital delivery on Zoom, using a combination of live digital classroom sessions and small group work. There are regular breaks throughout the day, a one-hour lunch break, and opportunities to ask questions and get feedback from your Trainers.
Please note: this course is a taster; it does not lead to qualification or recognised expertise in the profession.


What will we cover?
All topics are explored through interactive case studies.
Who should attend?
If this isn’t you, please consider recommending this course to your colleagues!
Where is this workshop taking place?
This workshop is delivered online via Zoom across one full day, including breaks.
Joining instructions will be emailed to attendee’s registered e-mail address, with a reminder sent shortly before the event.
Queries: If you are unable to complete your booking online or have any questions, please email training@fpm.org.uk.
Please see our Terms and Conditions and Privacy Policy.
Agenda:
9:15- 9:30 Welcome & Introduction
9:30- 10:00 Early development
10:00- 10:45 Interactive case study part 1
11:00- 11:45 Clinical development
11:45- 12:30 Interactive case study part 2
13:30- 14:15 Regulation
14:15- 15:00 Interactive case study part 3
15:15- 16:00 Medical Affairs and Pharmacovigilance
16:00- 16:45 Interactive case study part 4
16:45- 17:00 Wrap up and close
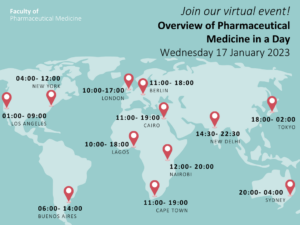
This workshop will take place on:
Date: Wednesday 17 January 2023
Time: 09:00-17:00 (UK time)
Price options:
FPM Member Rate: £250 £100*
Non-member rate: £350 £200*
This event is now sold out!
Please see our Terms and Conditions and Privacy Policy.
*Please note that these are special introductory prices for this event only. Future editions of this training will be charged at the standard rates.
Event disclaimer
Please note: this course is a taster; it does not lead to qualification or recognised expertise in the profession.
This training probably isn’t for you but please consider recommending it to your colleagues who are new to the field!
Sheuli Porkess:
Dr Sheuli Porkess is the Vice President of FPM, Chief Medical Officer for Precisia Life Sciences and Director of Actaros Consultancy.
Sheuli is an experienced pharmaceutical physician in medicine development, medical affairs and life sciences policy within the UK and internationally, across multiple therapy areas. Sheuli has held medical leadership roles in companies at a national, regional and international level whilst living in different countries and is the former Director of Research, Medical and Innovation at the Association of the British Pharmaceutical Industry.
Sheuli is a Fellow of the Faculty of Pharmaceutical Medicine, a Global Fellow in Medicine Development with IFAPP and a Fellow of the Royal College of Physicians of London and holds a number of other advisory roles. Sheuli currently delivers on FPM and ABPI’s Pharmaceutical Medicine Lecture Programme for medical students at Brighton and Sussex Medical School and Kent Medway Medical School. She also contributes to content and teaching on FPM’s DPM Training Programme.

Susan Tansey:
Susan is currently an Independent Consultant Pharmaceutical Physician. After training for several years in paediatrics and neonatology in the NHS, she joined the pharmaceutical industry and has since been involved in the clinical development of new medicines and vaccines in several therapeutic areas with a focus on rare diseases and paediatrics.
Susan also has an interest in bioethics and having been on the Nuffield Council of Bioethics’ Working Group on Children and Clinical Research between 2013 to 2015 was appointed as a Council member in 2020 and has just commenced as second term of appointment. She recently started to study for a part-time MA in Medical Ethics and Law at King’s College.
Susan is a Fellow of the Faculty of Pharmaceutical Medicine, a Global Fellow in Medicine Development with IFAPP and a Fellow of the Royal College of Paediatrics and Child Health. She currently delivers on FPM and ABPI’s Pharmaceutical Medicine Lecture Programme for medical students at Brighton and Sussex Medical School and Kent Medway Medical School. She also contributes to content and teaching on FPM’s DPM Training Programme.

Further study
For participants looking to undertake further study, there is the option of joining on of our classroom-based Physician and Scientist Induction Programmes.
This Programme is delivered across several days and will go into these topics in even further detail, whilst also providing an international perspective.
The programme is delivered by a team of pharmaceutical physicians and experts in the field, and participants will benefit by:
To register your interest, please contact training@fpm.org.uk.

Wednesday 23 April 2025
10:00 - 16:00
Find out more

Thursday 24 April 2025
Find out more

Thursday 8 May 2025 - Thursday 22 May 2025
Find out more

Tuesday 13 May 2025
09:15-17:00
Find out more

Thursday 5 June 2025 - Thursday 19 June 2025
Find out more

Wednesday 2 July 2025
11:30 - 17:00
Find out more
The views, information, or opinions expressed during FPM events and training are those of the individuals involved and do not necessarily represent those of the Faculty of Pharmaceutical Medicine. We value inclusivity, equality and diversity, and work hard to promote these whenever possible in all of our activities. We welcome your comments and feedback: events@fpm.org.uk