Investing for the Future
FPM Annual Report and Accounts for the year ending 31 December 2023

Forewords
From the President and the Chief Executive

As I complete the final year of my Presidency of the Faculty of Pharmaceutical Medicine (FPM), I look back on 2023 as another busy but productive year. The two priorities from my manifesto were focused on “Providing science support and medical education” and delivering “Robustness of growth, governance and financial stability”.
For the first priority, in 2023 there was an extensive external affairs programme to enhance profile of pharmaceutical medicine, which included campaigning for further investment in life sciences workforce and training, and collaborating through the Academy of Medical Royal Colleges (AoMRC) with the NHS, the GMC and UK Department of Health and Social Care (DHSC). In addition, we liaised with other life science organisations including the Academy of Medical Sciences, Medical Research Council, industry associations and medical societies. Workforce planning for training and resourcing has been a common theme, especially within the MHRA with whom we have discussed a significant collaboration.
Meanwhile, FPM has maintained its high profile in national consultations on topics ranging from genomics, AI, sepsis, COVID, antimicrobial resistance, rare disease and clinical trial recovery, to the FSRH Hatfield Vision project on equitable access to emergency contraception. We are also involved in policy-making for, and learnings from, COVID-19 and are engaging with the UK COVID Inquiry.
FPM education and standards has evolved extensively this year. NHS England have approved additional deanery support for the Pharmaceutical Medicine Specialty Training (PMST) programme and discussions are underway regarding implementation. We are also working towards being able to offer routes to GMC Registration for International Medical Graduates (IMGs) practising pharmaceutical medicine in the UK with a Postgraduate Qualification route for GMC Registration. We have also submitted to GMC our pharmaceutical medicine (PM) portfolio route for GMC Specialist Registration for IMGs.
In training, the digitisation of the Diploma in Pharmaceutical Medicine (DPM) Training Programme has been a great success and in house training offerings continue. FPM has also been discussing specific career rotations both within pharmaceutical medicine (e.g MHRA / industry) and also with other Higher Medical Training pathways in other disciplines (e.g. industry/clinical).
Membership is growing. You will have seen the updated eligibility criteria for FPM Fellowship to include those who are extensively qualified in PM and align with other college routes, and we are developing a Membership by Experience route in 2024 to attract as many experienced doctors in pharmaceutical medicine as possible. FPM is a GMC approved Designated Body and has seen its highest number of connected doctors since it began many of whom are Affiliate members.
The second priority “Robustness of growth, governance and financial stability” is critical to the long-term sustainability of our organisation. Operational performance in 2023 was challenged with fewer registrations for PMST, examinations and sponsorship income received was less than budgeted. In addition, there has been investment in staff and volunteers to modernise FPM. All this will ultimately generate significant operational income and efficiencies to stabilise the financial position. The Trustees are monitoring the situation closely to stem the income / expenditure deficit for 2024 to ultimately restore reserves and actively looking for other grants and funds. FPM staff, volunteers and Trustees have really worked hard in 2023 to underpin FPMs exciting future with many opportunities for a critical role in healthcare and life sciences.

Board of Trustees’ Report
The trustees are pleased to present their annual report together with the audited financial statements for the financial year ended 31 December 2023. The financial statements comply with current statutory requirements, the Memorandum and Articles of Association and the Statement of Recommended Practice – Accounting and Reporting by Charities (SORP FRS 102).
Our purpose
To advance the science and practice of pharmaceutical medicine by working to develop and maintain competence, ethics and integrity and the highest professional standards in the specialty for the benefit of the public.
Public benefit
The charitable purposes of Faculty of Pharmaceutical Medicine (FPM) are set out in the Memorandum and Articles of Association and are:
- to promote the science of pharmaceutical medicine.
- to develop and maintain competence, ethical integrity and high professional standards in the practice of pharmaceutical medicine; and
- to advance knowledge in pharmaceutical medicine.
Pharmaceutical medicine is the medical specialty concerned with the discovery, development, evaluation, licensing and monitoring of medicines and the medical aspects of their marketing.
FPM seeks through its activities to bring about an improvement in the health of the public and patients. Our activities seek to advance the science and practice of pharmaceutical medicine by contributing to the provision of effective medicines for public benefit. The trustees regularly review the aims, objectives and activities of the charity referring to the Charity Commission’s guidance on public benefit.
Our vision
A world where effective medicines meet the needs of patients.
Our mission is
To advance the science and practice of pharmaceutical medicine for the benefit of the public.
We will do this through three strategic pillars:
- Trust: FPM will be trustworthy by facilitating an increased understanding of the discovery, development and delivery of new medicines, vaccines and medical devices and the interface with public health.
- Sustainability: FPM will be sustainable by building an effective foundation to support our work.
- Relevance: FPM will be relevant to its membership and embrace the wider professional community in pharmaceutical medicine.
FPM’s values
How we deliver the strategy will be guided by our values, which underpin staff and members’ behaviour:
| We Are: | This Means: |
|---|---|
| Professional | This means being accountable for our work and actions |
| Innovative | This means we seek solutions proactively |
| Caring | This means we treat everyone with dignity |
| Collaborative | This means we work positively with others |
| Credible | This means we are honest and ethical in our work |
| Learned | This means we invest in developing knowledge and skills |
FPM Strategy 2023-2025
FPM launched its new Strategy 2023-2025, which is underpinned by three strategic pillars of Trust, Sustainability and Relevance. The first year of the strategy was focused on modernising the operations and digital assets of the business, as well as engaging more widely with external stakeholders.
Priority 1: Trust
Stakeholder engagement
The policy and communications function of FPM primarily exists to ensure our members are well supported, and are informed about and engaged with FPM activities, and to interact with external stakeholders, including: government; third sector bodies; the life sciences industry and regulators; colleagues in academia and clinical healthcare; the press; and patients and the general public. We continue to raise awareness of and advocate publicly for the science and practice of pharmaceutical medicine and the benefits that the speciality can bring to public health.
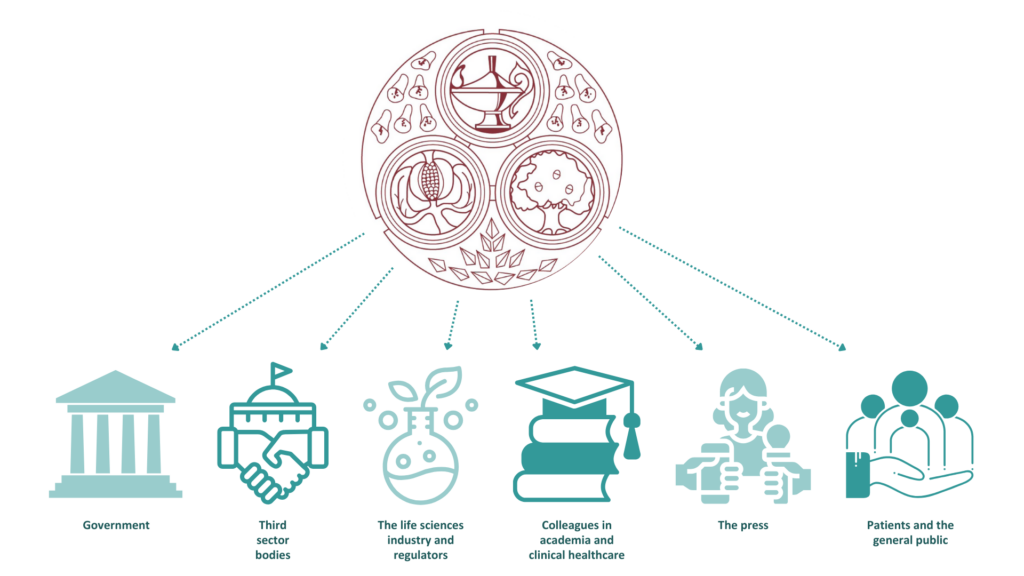
The FPM Policy and Communications Group (PCG) and seven ‘expert groups’ are comprised of members with a special interest and expertise in external engagement and extensive subject matter knowledge. The expert groups cover almost every aspect of pharmaceutical medicine, from therapeutics such as oncology, respiratory and infectious disease through to medical devices and other new technologies, to the regulations around the development and use of treatments. All these groups work alongside a professional staff team with expertise in digital content and strategy, editorial processes and public policy development.
In February, FPM hosted a roundtable in collaboration with MedCity and Imperial College Healthcare NHS Trust to address diversity in clinical trials. The resulting report, “Advancing Clinical Trials: Making the UK a global leader in inclusive and diverse clinical trials” made several recommendations which were referenced in the Lord O’Shaughnessy national review into the landscape for commercial trials in the UK.
Public policy in 2023
The PCG and expert groups monitor developments in science and policy, both in the UK and around the world. In 2023 they were particularly busy developing responses to several major public consultations, including: WHO guidance on clinical trials; the UK Government Major Conditions Strategy; the ICH Guideline for Good Clinical Practice; the DHSC consultation on supporting the delivery of COVID-19 and influenza vaccination; and the NICE guidance on the management of sepsis.
The infectious disease expert group continued their work reviewing guidance and supporting understanding of COVID-19 therapeutics and vaccines. Towards the end of 2023 this group also supported a major project to develop a suite of e-learning modules to support a better understanding of the symptomatology and treatment of common infectious diseases amongst healthcare professionals.
 FPM also continues to play a very active role in collaborative engagement across the healthcare and life sciences ecosystem. Our President has been leading a strand of work focusing on the reclassification of emergency contraception as part of the Faculty of Sexual and Reproductive Health’s ‘Hatfield Vision’ – that “by 2030, reproductive health inequalities will have significantly improved for all women and girls…”. The FPM Vice-President and Chief Executive have been involved in multiple strands of work concerned with addressing health inequalities, and FPM was also a joint signatory to the “Advancing Clinical Trials: Making the UK a global leader in inclusive and diverse clinical trials” report. FPM is also an active member of the UK Health Alliance for Climate Change.
FPM also continues to play a very active role in collaborative engagement across the healthcare and life sciences ecosystem. Our President has been leading a strand of work focusing on the reclassification of emergency contraception as part of the Faculty of Sexual and Reproductive Health’s ‘Hatfield Vision’ – that “by 2030, reproductive health inequalities will have significantly improved for all women and girls…”. The FPM Vice-President and Chief Executive have been involved in multiple strands of work concerned with addressing health inequalities, and FPM was also a joint signatory to the “Advancing Clinical Trials: Making the UK a global leader in inclusive and diverse clinical trials” report. FPM is also an active member of the UK Health Alliance for Climate Change.
FPM recently supported the appointment of a new pharmaceutical physician to sit on the BMA’s Medical Academic Staff Committee, which is the ‘home’ to pharmaceutical medicine within the BMA.
In late 2023 FPM formed a new group, the Working in Partnership with Patients & Communities Forum. This new forum aims to leverage FPM’s influence to support and champion active partnerships with patients and communities in the healthcare and life science sector and to ensure patient advocacy is central to all of FPM’s work. The forum hosted an open, free-to-attend, webinar on Embedding a Patient Centric Approach and then an in-depth FPM Masterclass on The Fundamentals of Patient Engagement for Pharmaceutical Physicians. They have also developed a training session for FPM staff on patient engagement, the outcome of which will be the development of internal guidance and policies on best practice.
FPM collaborated with Liquona, an award-winning video production agency, to curate and launch a new animation to promote the pharmaceutical medicine specialty programme (PMST) as a route to professionalism in pharmaceutical medicine. fpm.org.uk/blog/new-animation/
FPM secured £145k of grant funding to develop four e-learning
modules on respiratory diseases to be launched in 2024, building upon
the recommendations of the Faculty’s DEMENDE (DEfining MEdical NeeDs and Evidence) report in 2022. A suite of e-learning modules for healthcare professionals will be produced to support their understanding of the symptomatology and treatment of common respiratory infections.
FPM collaborated with RCP London and other bodies to develop the e-learning module, “Research in practice: Getting involved in clinical research”, which went live in June 2023. The module is available on the NIHR Learn platform. As of July, some 100 people had accessed one or more of the modules, initial feedback was very positive and discussions in the autumn focused on how the material might be made available to the widest possible audience.
In the UK, the teaching of how medicines are developed and used is not included in the majority of undergraduate teaching or postgraduate training for clinical doctors and other allied healthcare professionals (HCPs) who work in the NHS. Medicines are used throughout the NHS and providing education to help all doctors and HCPs to learn how medicines are developed, regulated and accessed will contribute to greater understanding and trust. This was one of the reasons why the undergraduate Drug Discovery and Development programme was developed in conjunction with the ABPI and Brighton and Sussex Medical School. The programme entered its fourth year in 2023 and is delivered also to Kent and Medway Medical School. The success of the programme has led to plans to develop a digital version which can be delivered to a wider number of medical schools on how medicines are created and accessed.
The Physician and Scientist Induction Programme was another success story this year, with the third and fourth sessions delivered for a US-based client in May and November respectively. Our small portfolio of one-day training courses also attracted healthy numbers, not least for the popular “ABPI Code in a Day” course, which ran four times in the year.
FPM event and training attendees
155
FPM Annual Symposium
18
In-company training
49
Code in a Day
82
FPM Education Day
76
DPM Training Programme
59
FPM Conversations
The FPM Designated Body
FPM is a Designated Body (DB) for providing annual appraisals and GMC revalidation. At 31 December 2023, 690 members had a prescribed connection to FPM as a designated body, the highest number of connected doctors since the introduction of revalidation at the end of 2012.
During the 2023/24 appraisal year, 83 doctors connected and 61 disconnected.
Guidance continues to allow appraisals to take place via video conference. The GMC have published their new Good Medical Practice standards during the year, their first update for ten years. This will come into effect on 30th January 2024. The FPM DB commenced its preparations for its introduction into appraisals and this will continue in 2024.
We are fortunate to have 80 highly enthusiastic and competent appraisers. Of these, we have 20 appraisers who are connected to other designated bodies including, for example, the NHS. This helps to ensure similar standards across pharma and benchmark with other designed bodies. We have continued to hold quarterly meetings with the Appraisal Leads which are valuable for ensuring consistency and for generating quality improvement ideas. The Appraisal Leads also deliver an online introduction to appraisal and revalidation every six weeks, mainly for newly connecting doctors but it is open to all connected doctors. During the year, we provided four networking sessions for our appraiser team. Attendance at the non-mandatory sessions was excellent with 73% of appraisers attending.
Although our lay advisor, Mr William Payne, has come to the end of his term as a Board Trustee he has agreed to continue as our Lay Representative. We have valued his wise counsel on a number of matters. The most recent ratings and free text comments from the post-appraisal questionnaire which appraisees complete are once again overwhelmingly positive. This includes views on the appraisal platform PReP and personal feedback on the appraiser. The results are aggregated for each appraiser and sent to them annually. Importantly they show that doctors felt well supported by their appraisers and the office team. It was clear that many felt that having the opportunity to discuss the events of the year with a peer was invaluable.
Priority 2: Sustainability
Working towards the long-term sustainability of FPM was a key driver in 2023, with a focus on strengthening operational resilience. FPM invested in its digital infrastructure to streamline its processes, as well as strengthen income streams through four major digital change projects:
- Implementation of the Learning Management System (LMS), known as the FPM Learning Hub
- Transformation of DPM Training programme
- Replacement of Customer Relationship Management system (CRM)
- Commissioning a new self-service webportal
Modernising its digital infrastructure enables FPM to expand the provision of postgraduate medical education to meet growing demands for professional and technical training on line as well as deliver a broader diversity of events and engagement opportunities for members as well as the public.
FPM successfully launched its new Learning Management System which was designed and delivered with the developer Synergy LMS. The new Microsoft Dynamics CRM and webportal were developed and delivered by Bluelight to provide a modern integrated system which enhances the user experience, automates administrative processes as well as support the provision of improved data analytics, contact management and bespoke reporting.
Ensuring that pharmaceutical physicians are properly trained and educated helps to build trust with the profession. This means that it is important that FPM delivers training and education that is accessible, inclusive and relevant to the needs of learners. This has been demonstrated through the redevelopment of the DPM Training Programme for asynchronous online delivery. The programme which launched successfully in March enables delegates from across the world to access the training programme at a time and place that is convenient for them. Registrations and income for the programme exceeded expectations in 2023, feedback on the programme was very positive and a plan is underway for additional enhancements to the materials for 2024.
Priority 3: Relevance
FPM continues to raise awareness of and advocate publicly for the science and practice of pharmaceutical medicine and the benefits that the speciality can bring to public health. 2023 has been a year of external engagement and collaboration. External engagement has occurred with a number of influential bodies including Academy of Medical Royal Colleges, the Department of Health and Social Security, the Academy of Medical Sciences, the Office of Life Sciences and the National Institute for Health and Care Research (NIHR).
An important engagement was with Faculty of Sexual & Reproductive Healthcare (FSRH) which resulted in FPM endorsing its strategy called the “Hatfield Vision for Improvements in Sexual and Reproductive Health”. The FPM President was invited to co-chair the Emergency Contraception Implementation Working Group meetings as FPM’s expertise was recognised and needed.
The Working in Partnership with Patients & Communities Forum was launched in autumn 2023. This new forum aims to leverage FPM’s influence to support and champion active partnerships with patients and communities in the healthcare and life science sector and to ensure patient advocacy is reflected in FPM’s work.
FPM is an active member of the Inequalities in Health Alliance and the UK Health Alliance for Climate Change, both of which address the social as well as environmental impact of ongoing disparities to access medicines and healthcare.
The UK media was dominated by prominent figures delivering evidence to the COVID-19 Inquiry. FPM received a notice to provide evidence to Module 4 (vaccines and therapeutics) as well as contributed to the AoMRC’s evidence for Module 3.
Supported by its expert groups, FPM has responded to several major public consultations, including WHO guidance on clinical trials, the UK Government Major Conditions Strategy, the NICE Single Technology Appraisal on AZD 3152 for preventing COVID-19, the ICH Guideline for Good Clinical Practice, the DHSC consultation on supporting the delivery of COVID-19 and influenza vaccination, and the NICE guidance on the management of sepsis.
FPM delivered to two core events in 2023. The Education Day in June explored the theme, “Brave New Worlds” and speakers examined a host of innovations in drug development which are expected to enable faster access to new, life changing treatments.
The Annual Symposium theme of “Navigating the future-scape of pharmaceutical medicine” examined some of the biggest challenges and most exciting opportunities for global healthcare in the post-pandemic world.
One of the best symposiums I have attended.
Much appreciate the organisation – a massive task.
It was a thoroughly enjoyable event and clearly a great deal of thought and preparation went into the meeting.
Photographs from the 2023 editions of FPM Annual Awards, FPM Education Day, and FPM Annual Symposium
Equality, Diversity and Inclusion
FPM’s Equality, Diversity and Inclusion (EDI) Forum continued work towards its vision of ensuring that FPM is diverse and inclusive at every level of the organisation, whilst members continued to act as champions for EDI in their own professional and personal capacities outside of FPM. Under the chair, Dr John Ndikum, the EDI Forum has undertaken a wide range of activities raising awareness of EDI-related issues through multiple Fireside Chats with prominent organisations and speakers, including Steve Fuller and BlackPharma, blog posts, including a fascinating piece on highlighting the importance of patient care and striving for better outcomes by Dr John Bolodeoku, and generating content for FPM’s social media channels for important World Days.
Digital Communications
FPM’s digital communications strategy and activities went from strength to strength during 2023. A particular focus was put on telling the stories of pharmaceutical physicians’ careers, notably through a small but growing library of careers videos developed in collaboration with the careers working group. These and other outputs have been shared with audiences using three digital comms strands:
Strand one is the website fpm.org.uk. We have focussed on the regular publication of blogs – commentary pieces, analyses and reflections on the latest scientific developments, changes to regulations and the views of patients. We have also rolled out incremental updates to the design to enhance the user experience. The current website, launched in March 2020, continues to perform well and has sustained high traffic volumes despite fewer blog articles being published in 2023.
Strand two is email communications. The monthly FPM Bulletin collates blog articles, internal and external news, event information and much more into an easily digestible and engaging update. The FPM Bulletin is complemented by additional distinct campaigns spotlighting events, opportunities to get involved, and the latest blogs. Open rates of the monthly FPM Bulletin campaigns averaged 53.7% which is far above benchmarks.
Open rates (%age) of email campaigns (source, Mailchimp (2024)
Strand three is social media. The FPM LinkedIn page remains a key tool in building our audience and our brand amongst established and new markets. This audience has been developed organically with a content-oriented publication strategy and in 2023 our page followers increased by 19% to more than had more than 6,800. Posts also saw an average engagement rate of 1.45%, which is 20% more than the average for professional services (source: Hootsuite, 2024). Particularly popular LinkedIn posts during 2023 included the careers videos which have cumulatively been viewed several thousand times, as well as posts that celebrated significant achievements of our members.
Outside of LinkedIn, FPM’s digital achievements during 2023 included a >100% increase in YouTube channel subscribers, and the launch of a brand new Instagram account. With more than 1.2 billion monthly active users, Instagram was – until September 2023 – the largest social platform without an FPM presence. A distinctive sub-brand was developed for the image-oriented platform, and a schedule of content is now delivered to at an audience that is younger and/or less aware of medicines development than existing audiences.
Sample Instagram posts Designs use established brand colours to ensure familiarity.
FPM Celebrates
FPM Annual Awards
The FPM Annual Awards took place in June 2023 at the RCP London and gave us an opportunity to celebrate achievements of members and other colleagues in the life sciences who have made significant contributions to the field of pharmaceutical medicine.

The FPM President’s medal, which is the highest honour FPM bestows, was awarded to Dr Ruth Dixon. Dr Dixon has been a leading light in the education of pharmaceutical physicians for over two decades, thus helping to set the standards for the professionalism, commitment, and integrity of the next generation of pharmaceutical physicians.
The FPM Volunteer Award went to Dr Susan Tansey and the academic achievement awards, given to the candidates who scored the highest marks in the DPM Part 1 and DPM Part 2 examinations respectively, were both awarded to Dr Arash Yavari.
Honorary Fellowships were bestowed on Dr Howard Freeman, Professor Sir Mene Pangalos, Professor Dame Helen Stokes-Lampard and Professor Martin Wilkins.

External Recognition
The President, Dr Flic Gabbay, was awarded an Honorary Fellowship by the Faculty of Public Health at their award ceremony in July.
The Chief Executive, Dr Marcia Philbin was awarded an honorary doctorate by her alma mater, Aston University in Birmingham where she had studied for both her degree and PhD in chemistry. The award was in recognition of her achievements and commitment to championing for greater diversity in science. In addition, Dr Philbin, was invited to deliver the prestigious annual MacLaren Memorial lecture by the Chartered Management Institute. The MacLaren Memorial Lecture, an annual event which has been held since 1953, commemorates the life of James MacLaren, one of the most eminent industrialists in Birmingham’s history.
Future Plans
A priority in 2024 will be on improving the financial reporting in 2024, with better monitoring and controls in place. FPM has recruited a chartered management accountant with over 30 years’ experience to implement new processes which will provide more visibility to emerging situations to which FPM can respond. The plan in place will ensure that FPM strengthens its financial resilience by 2026.
There will be a continuation of the modernisation of FPM with a focus on strengthening processes and income streams along with continuing to engage stakeholders. A new President whowas elected in February 2024 will advocate for the medical specialty nationally and globally with a particular focus on addressing the future needs for training, education and policy.
In terms of processes, the priority will be on reforming the governance and operational delivery of the pharmaceutical medicine specialist training programme to ensure a clear delineation of responsibilities. A review and revision of the examination question and standard setting processes will be undertaken to enable FPM to deliver at least two diets of examinations per annum from 2025. Another important development will be to modernise the criteria for Membership and Fellowship so that FPM is open to qualifying physicians who meet the relevant standards.
Efforts will be directed also at securing funds to develop the undergraduate training programme into a series of eLearning modules which will be accessible to medical schools in the UK.
In terms of informing the development of future health policy, FPM will continue to submit evidence to the COVID-19 inquiry to illustrate the importance of pharmaceutical medicine and its vital role in a pandemic and how FPM can support improvements to the system.
2024 will see FPM deliver against its priorities of trust, relevance and sustainability to ensure pharmaceutical physicians are educated and trained to address the future challenges which they will encounter in a world that is changing fast and where the demands for healthcare solutions will increase.
Thank You
Finally, once again FPM would like to extend thanks to all our members who contributed to our activities in 2023, whether as committee members, examiners, specialty advisers, educational supervisors, appraisers or by supporting raising awareness and advocacy events and policy projects. We truly value your participation and support.











